China COVID-19 Vaccine Tracker
Chinese leaders have repeatedly stated that China will make its COVID-19 vaccine a global public good. More recently they declared that China is indeed fulfilling this pledge by exporting and donating its COVID-19 vaccines to countries around the world. This has generated a great deal of interest and discussion around the world. On June 2, 2021, China’s Ministry of Foreign Affairs stated that China has provided “more than 350 million doses of vaccines to the international community, including vaccine assistance to over 80 countries and vaccine exports to more than 40 countries”. As an independent, mission-driven consultancy that tracks China’s impact on global health, Bridge aims to examine and offer a comprehensive picture of China’s vaccine outreach, which will hopefully enable more informed discussions on this issue around the world. This vaccine tracker is based fully on data from reliable publicly accessible sources collected and compiled by our team and is updated on a weekly basis.
758M DOSES SOLD
23M DOSES DONATED
272M DOSES DELIVERED
Updated as of 12:00 pm (GMT+8), June 15, 2021.
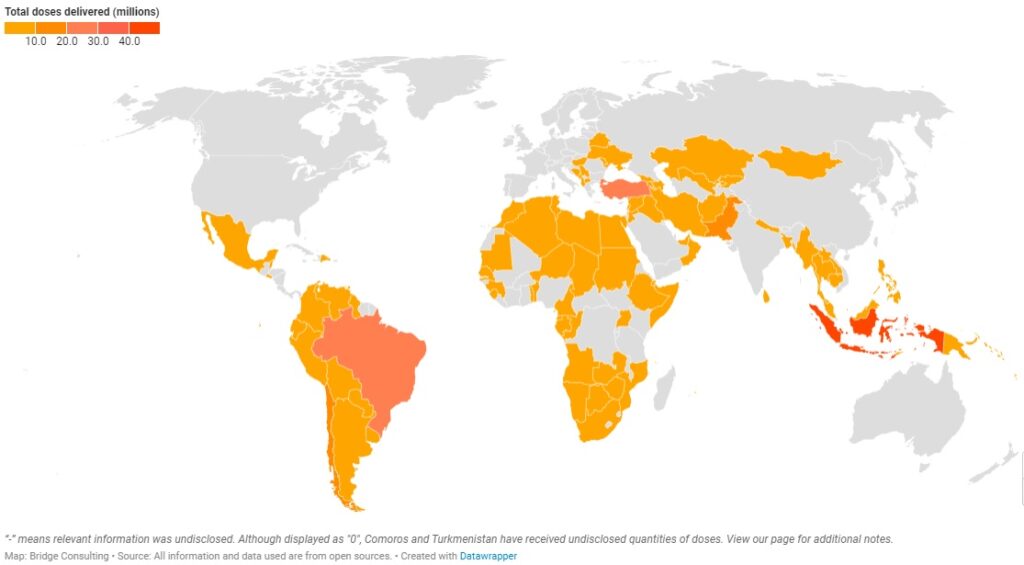
China’s Vaccines Around the World
This interactive map tracks the vaccine sales and donations China and Chinese vaccine developers have made as well as the number of vaccines delivered by China.
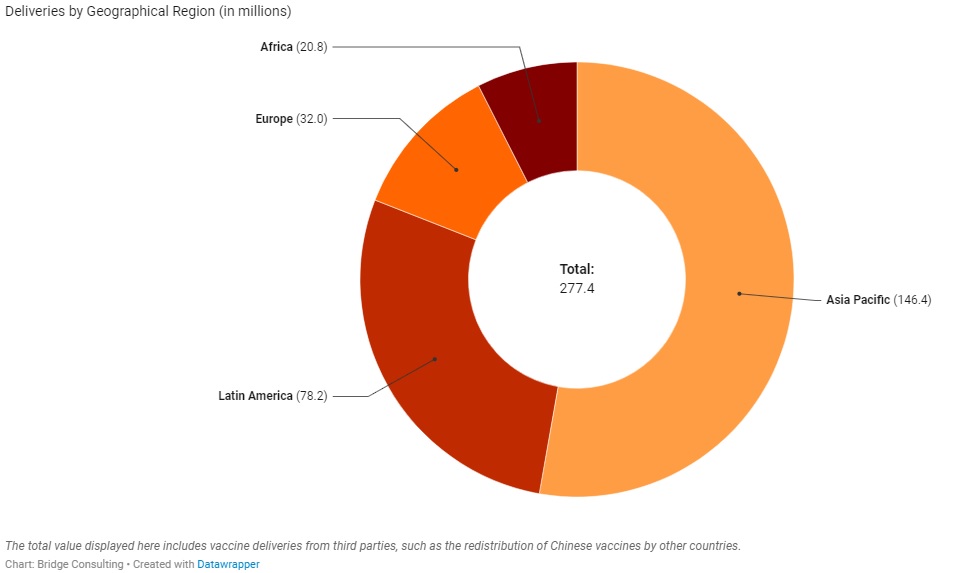
China’s Vaccines Across Regions
China has provided vaccines to four geographical regions – a total of 95 countries around the world. Out of the four regions, Asia Pacific has received the greatest number of Chinese vaccines, with 35 countries receiving sales and donations from China. Latin America, however, has received the second greatest number of Chinese vaccines with only 18 countries receiving sales and donations. In contrast, Africa has 32 countries receiving sales and donations from China, but it has received the least number of Chinese vaccines.
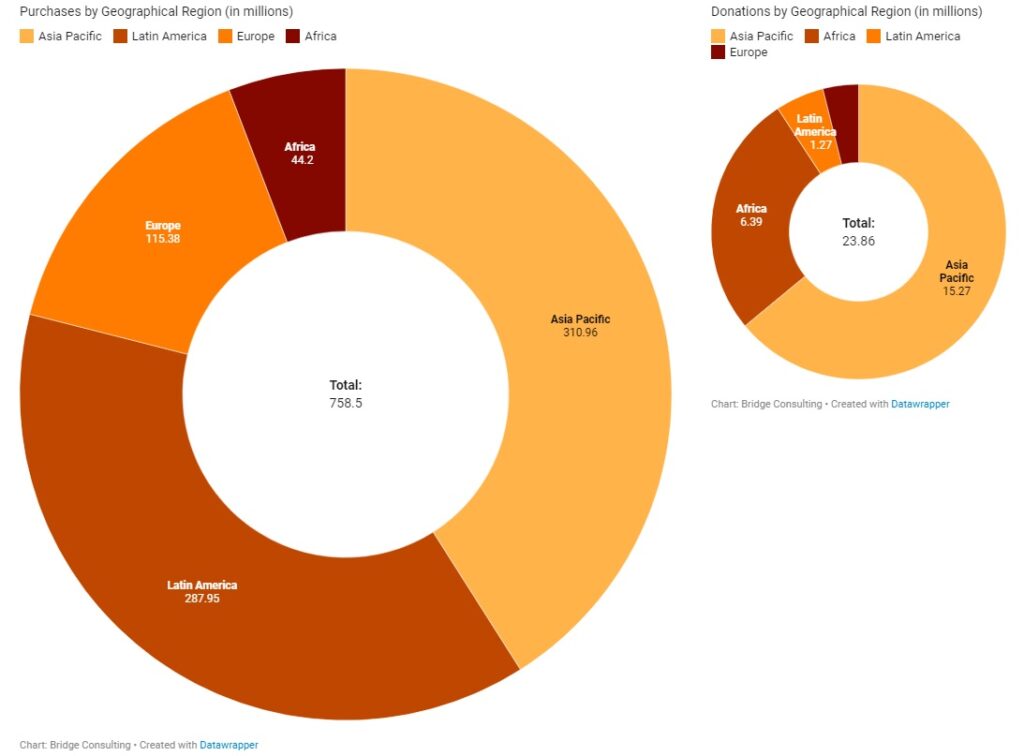
The quantity of vaccines sold by China is more than 30 times that of its donations, with Asia Pacific and Latin America by far the two biggest buyers of Chinese vaccines. Europe, on the other hand, has had relatively fewer deals with China. Africa is the second largest receiver of Chinese vaccine donations worldwide, but vaccine deliveries to the region remain the lowest.
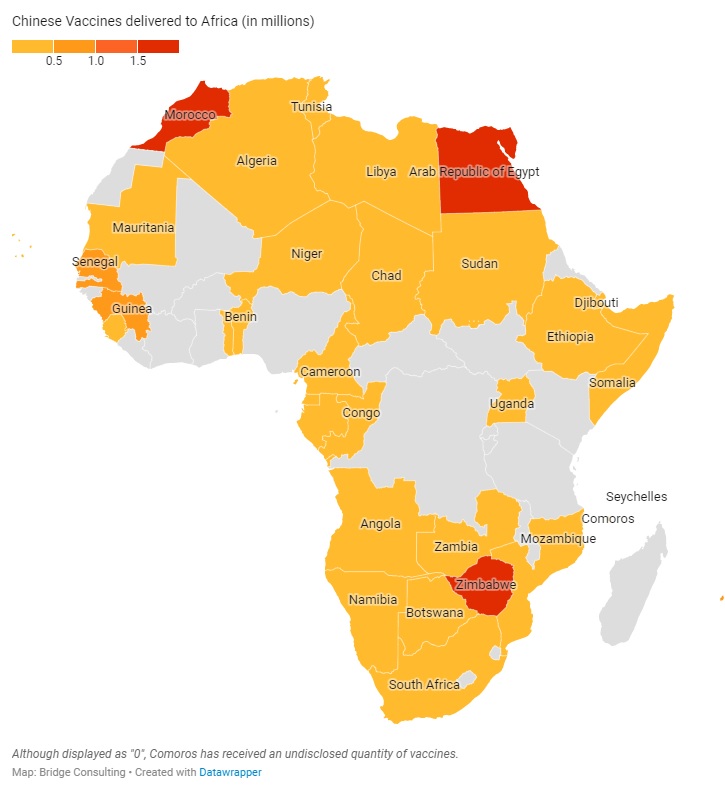
CHINA’S VACCINES IN AFRICA
As part of the South-South Cooperation, China pledged in late February to provide COVID-19 vaccines to 19 African countries. To date, 33 African nations have been receiving sales and donations of vaccines from China. In May, the pace of these deals had picked up; however, the total number of vaccines delivered to Africa by China has remained lowest among the regions. Overall, China’s vaccine outreach in Africa still appears to be rather slow.
Alongside bilateral agreements, Africa has also been receiving vaccines through the COVAX initiative. China has delivered a total of 20 million doses to Africa, out of the 44 million pledged. In contrast to other regions, Africa has been receiving Chinese vaccines through more donations than sales. Issues of affordability and accessibility are particularly large for African countries with limited financial resources at their disposal.
Chinese leaders have vowed to increase the affordability and accessibility of COVID-19 vaccines in Africa. Indeed, China has donated 6 million doses of vaccines to African countries, but more should be done to help African countries with urgent needs to obtain safe, effective, and affordable COVID-19 vaccines without delay.
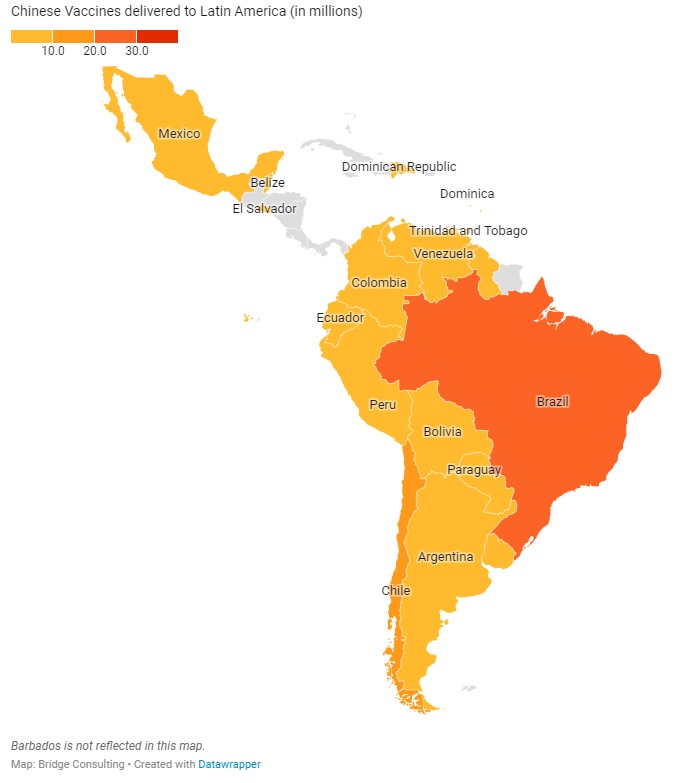
CHINA’S VACCINES IN LATIN AMERICA
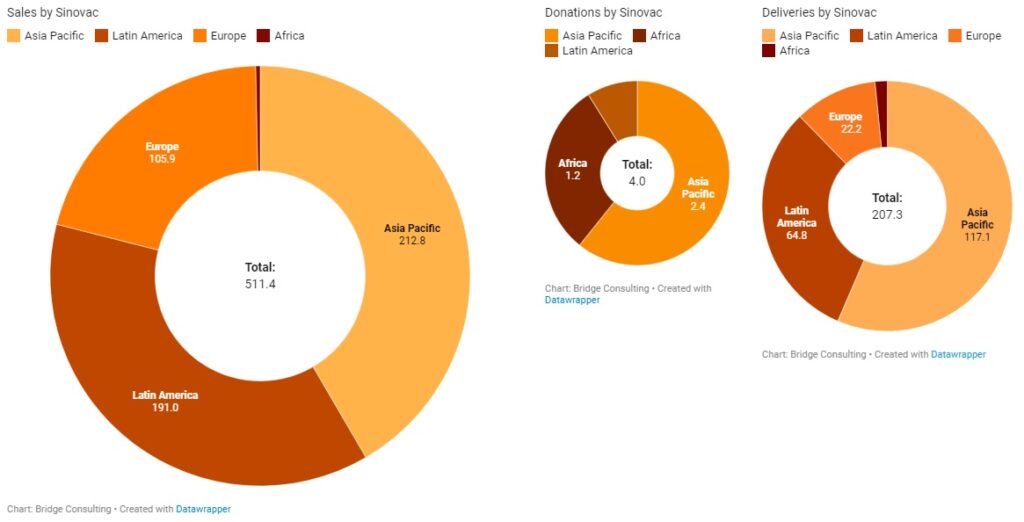
Latin America has received the second largest quantity of Chinese vaccines, despite having only 18 countries with vaccines deals with China. Latin America’s relations with China have always been close, especially since both regions are also working under the South-South Cooperation and the Belt and Road Initiative. China has donated only 1 million vaccine doses to the region, and has sold 287 million doses, with 78 million doses delivered. China is also providing the region with active ingredients to make other vaccines, such as the AstraZeneca vaccine.
Latin America plays an especially significant role to Chinese vaccine developer Sinovac, which has sold 191 million doses (out of 511 million doses sold globally) to 9 Latin American countries. Amid the ongoing debate over the use of its vaccine in the region, Sinovac is increasing its efforts to improve its image in Latin America, which is best demonstrated by its decision on April 13 to independently donate 50,000 vaccine doses to the South American Football Confederation for South American countries’ national football teams to use ahead of the South American Football Championship (also known as Copa America), one of the largest and most-watched sporting events in Latin America and the world.
Brazil
Brazil is the largest buyer of Chinese COVID-19 vaccines in the region, having purchased 100 million doses of Sinovac vaccine. Since October 2020, from when Sinovac was conducting Phase 3 trials in the country however, Brazil’s President Bolsonaro has had contradicting opinions on Chinese vaccines. Phase III results released by Brazil’s Butantan Institute show the Sinovac vaccine has a 50.7% efficacy at preventing symptomatic infections, and 83.7% effective in preventing mild cases needing treatment, which has prompted debates on the use of Sinovac vaccine.
Unlike many countries which mainly use ready-to-use Sinovac vaccines shipped from China, Brazil is also manufacturing Sinovac vaccines locally using active ingredients sent from China. The state-run Butantan Institute is now filling and finishing Sinovac vaccines in Brazil, with plans for 100% domestic production in early 2022. The Butantan Institute is also building a facility to produce 100 million doses a year of China’s Sinovac COVID-19 vaccine, which will be ready by September 2021.
Additionally, on April 19, Brazilian health regulator Anvisa authorized the phase 2 and 3 trials of a new COVID-19 vaccine developed by China’s Sichuan Clover Biopharmaceuticals. Our team has been closely following the development of Clover’s COVID-19 vaccine. Based on results published on the Lancet, Clover’s vaccine candidate shows promising results. Clover’s vaccine R&D has been supported by Coalition for Epidemic Preparedness Innovations (“CEPI”), which co-leads the COVAX Facility alongside Gavi, the Vaccine Alliance and the World Health Organization (WHO). CEPI already stated that COVAX hopes to procure hundreds of millions of doses of Clover’s vaccine if proven to be safe and effective. Clover’s vaccine could give Brazil and the rest of the world another effective tool to combat the COVID-19 pandemic.
Chile
Chile is the third largest buyer of Chinese vaccines in the region. The Chilean government has purchased 60 million doses of Sinovac vaccines in a three-year deal for its 19 million people. Amid recent debates over efficacy rates of Chinese vaccines, Chilean authorities backed the country’s widespread use of the Sinovac vaccine, having largely relied on the it, along with smaller numbers of Pfizer’s. Chile has one of the fastest vaccination campaigns in the world and 86.9% of vaccines it has are the Sinovac vaccine.
On April 16, Chile’s health ministry released a study of its vaccination campaign so far, which showed the Sinovac vaccine was 67% effective at preventing symptomatic infection, and more than 80% effective at preventing hospitalizations and deaths. The report did note the Sinovac vaccine was only 16% effective against infection after one of the two doses required. The Chilean government is shifting its vaccination strategy toward issuing second doses because of this result and concerns over supply shortage.
Mexico
Mexico is the second largest buyer of Chinese vaccine in the region. It is the only country in Latin America to have deals for Sinovac, Sinopharm and CanSino vaccines. In February 2021, Mexico became the first country in the world to approve China’s CanSino vaccine for emergency use. The country also uses locally packed CanSino vaccines that are received in bulk from China.
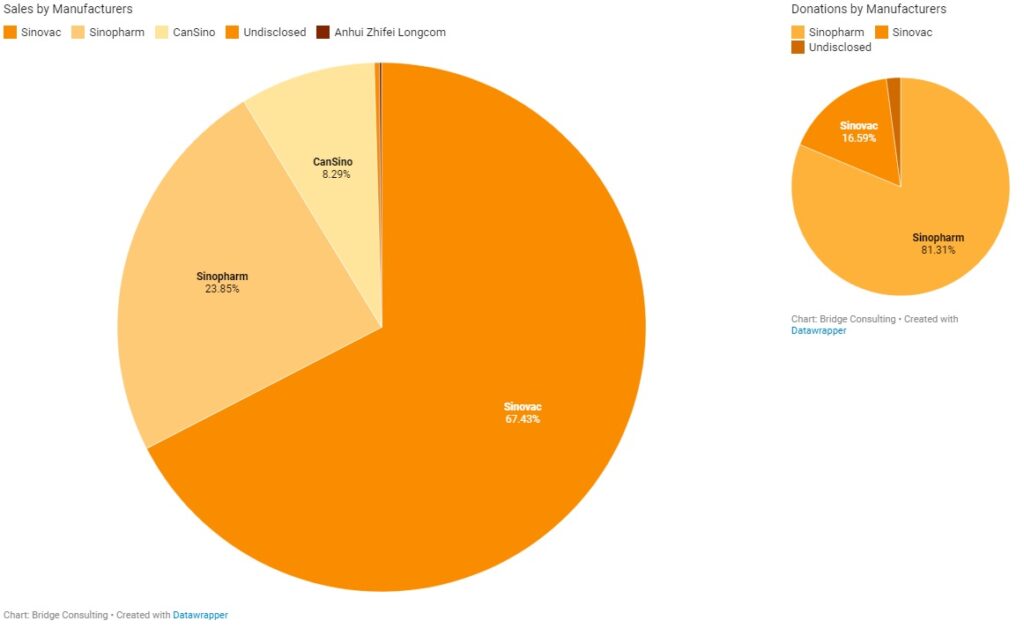
China’s Vaccines by Manufacturers
Sinopharm and Sinovac have been the main manufacturers of the COVID-19 vaccines sold and donated by China. China’s total vaccine sales stand at 758 million doses to date. Sinovac remains the main supplier for vaccine sales by China, having sold 511 million doses and supplied vaccines to 37 countries in total. China’s major deals with Indonesia, Brazil, and Chile, where manufacturing plants are also being set up, have been supported by Sinovac. It is also worth noting that Sinovac conducted trials in Indonesia, Brazil, and Chile, and these three countries purchased 140 million, 100 million, and 62 million doses of Sinovac’s vaccines respectively, after the vaccine was proven safe and effective.
On the other hand, Sinopharm has been the main supplier for vaccine donations from China, having supplied vaccines to a total of 68 countries. Total vaccine donations from China stand much lower at 23 million doses. Countries that have purchased vaccines from China appear to prefer Sinovac’s vaccines, while China appears to prefer Sinopharm vaccines for donations. Sinopharm conducted trials in countries including the United Arab Emirates, Egypt, and Morocco, which have purchased 18 million, 20 million, and 10 million doses of Sinopharm’s vaccine respectively.
Clinical trial locations appear to have a significant influence on vaccine orders. Other Chinese vaccine manufacturers have sent relatively small quantities of vaccines to other countries, but in recent weeks, these manufacturers are catching up by delivering more vaccines overseas and helping other countries set up overseas production facilities, such as CanSino.
SINOVAC – CORONAVAC COVID-19 VACCINE
On June 1, the World Health Organization officially listed the inactivated COVID-19 vaccine developed by Chinese biopharmaceutical company Sinovac Biotech Ltd. for emergency use. This is the second Chinese COVID-19 vaccine to receive the approval, after the inactivated Sinopharm COVID-19 vaccine was approved for emergency use in May. While there have been multiple studies done on the efficacy of this vaccine in different countries, WHO has stated that overall results have shown that the vaccine prevented symptomatic disease in 51% of those vaccinated and prevented severe COVID-19 and hospitalization in 100% of the studied population. Sinovac is the fastest growing Chinese vaccine manufacturer, in terms of production capacity expansion outside China
SINOPHARM – BEIJING BIO-INSTITUTE OF BIOLOGICAL PRODUCTS CO-LTD. (BBIBP) COVID-19 VACCINE
On May 7, the World Health Organization officially listed the inactivated COVID-19 vaccine developed by Beijing Bio-Institute of Biological Products Co-Ltd. (BBIBP) under the China National Pharmaceutical Group Corporation (Sinopharm) for emergency use, marking a major milestone as the first Chinese COVID-19 vaccine to receive this approval. Issues such as accessibility and affordability of the Sinopharm’s vaccine in developing countries still need to be addressed, but its advantages such as its less stringent storage requirement and expanding production capacity in and outside China will help countries and international organizations resolve some of the urgent problems facing the current global vaccine supply.
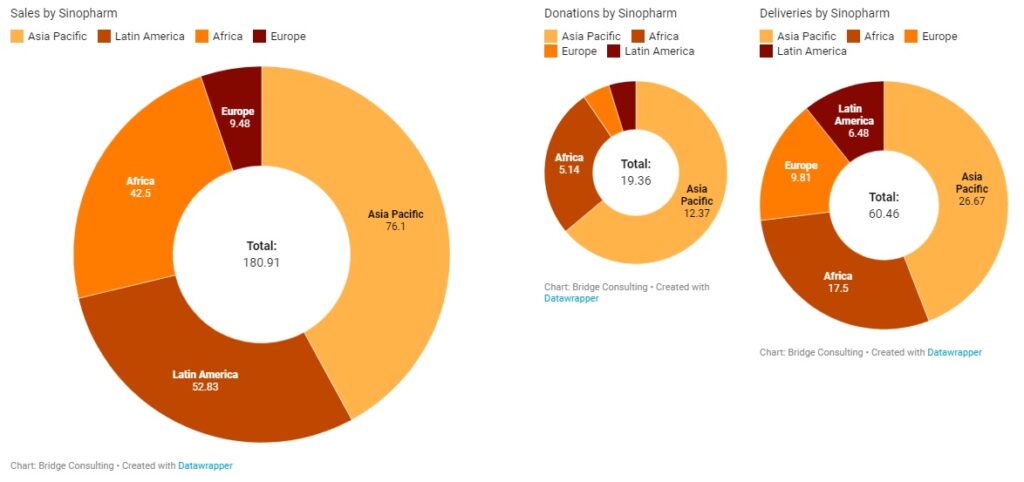
China’s Multilateral Vaccine Contributions
Announcements of China’s vaccine donations to the United Nations Peacekeepers and the International Olympic Committee marked the first wave of multilateral vaccine assistance in with Chinese leaders repeated emphasis. Chinese leaders have repeatedly emphasized that China uploads multilateralism and wants to promote multilateral cooperation in the global response to COVID-19. While China has committed 10 million doses of its COVID-19 vaccines to the COVAX Facility, and numerous Chinese vaccine developers have announced their intentions to supply their vaccines to COVAX, the vast majority of vaccines donated and exported by China so far have gone through bilateral channels. The Africa Centers for Disease Control and Prevention is also in an “advanced stage of discussion” to acquire the Sinopharm vaccine.
More recently on May 21, China announced a donation of 200,000 doses of COVID-19 vaccines and USD 1 million to the United Nations Relief and Works Agency for Palestine Refugees in the Near East (UNRWA) to help the Palestinians. China sending its vaccines to other countries is indeed encouraging, but in order to make the Chinese COVID-19 vaccine a global public good and promote multilateralism, China should increase its participation in vital multilateral mechanisms.
Overseas Manufacturers of Chinese Vaccines
On May 21, Egypt received its first batch of components to locally produce the Sinovac vaccine. The 1,400 liters of bulk vaccines will be made into 2 million doses of vaccines, which will be used for vaccination in Egypt in the next two months. So far, all overseas manufacturing facilities of Chinese vaccines use or plan to uses active ingredients or vaccines in bulk imported from China, which means these overseas facilities will only fill the vials and package the vaccines. It is worth noting that local media reported that the agreement between China and Egypt also includes transferring of production and manufacturing technology to enable local production of ingredients for the Sinovac vaccine in the future. Other countries producing or planning to produce Chinese COVID-19 vaccines such as Pakistan have similar plans to manufacture Chinese COVID-19 vaccines locally via technology transfer.
Many developing countries want to produce more COVID-19 vaccines for their population themselves. More than 100 countries, mostly developing countries, have asked the World Trade Organization members to agree to a time-limited lifting of COVID-19-related intellectual-property rights so more countries can produce vaccines for their own population. On the other hand, some countries argue such measures may not speed up manufacturing or supply and it would take more than a year to set up new production capacity even if patents did not apply. Experts have also advocated for COVID-19 vaccine developers to increase the licensing of their vaccines, which would allow vaccines to be made by many manufacturers around the world. Developed countries such as the US have voiced their support of a wavier on vaccine patents, while others such as those in the European Union have expressed reservations.
It is encouraging to see these efforts from Chinese COVID-19 vaccine developers to help their partners in developing countries to build up vaccine production capacity. Recently on May 21 at the Global Health Summit, Chinese President Xi Jinping reiterated China’s support for its vaccine developers to transfer technologies to other developing countries and carrying out joint production. He stated that “having announced support for waiving intellectual property rights on COVID-19 vaccines, China also supports the World Trade Organization and other international institutions in making an early decision on this matter”.
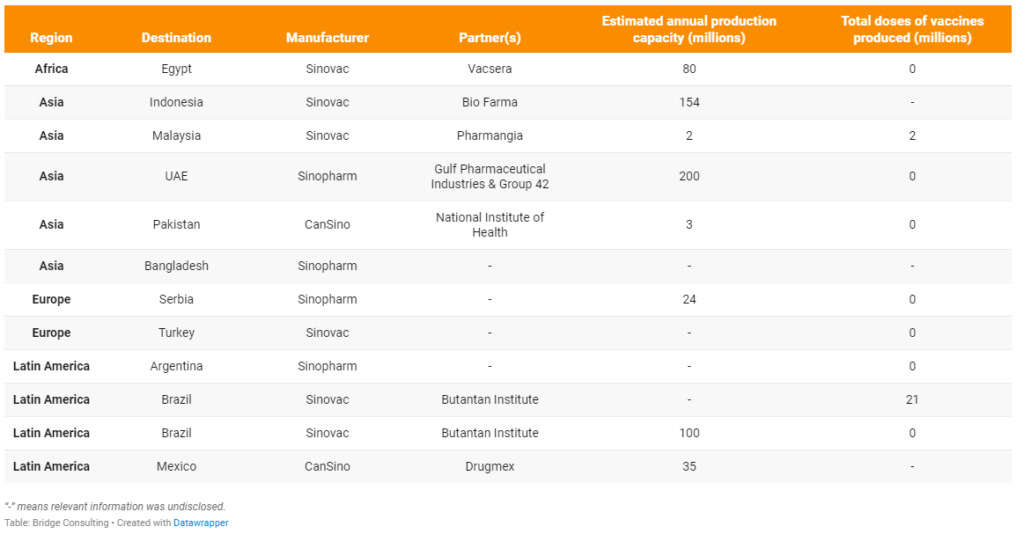
Further details:
Egypt. Egypt will start producing the first batch of the Sinovac vaccine within two weeks, coinciding with the Sinovac vaccine obtaining the approval of the World Health Organization. Sinovac will provide technical support and assistance to Vacsera in building and equipping manufacturing facilities to meet the required standards.
Indonesia. Indonesia has received 81.5 million vaccines in bulk as of June 1, 2021, and Bio Farma is aiming for producing 154 million doses before the end of the year.
Malaysia. On April 23, 2021, the “fill and finish” Sinovac vaccine by Pharmaniaga received conditional approval from Malaysia’s National Pharmaceutical Regulatory Agency (NPRA), allowing its use for mass vaccinations.
United Arab Emirates. The new plant in the UAE, which is being built in the Khalifa Industrial Zone of Abu Dhabi (KIZAD), will have a production capacity of 200 million doses a year with three filling lines and five automated packaging lines, a statement from the joint venture said on Monday. Vaccine manufactured in UAE will be called Hayat-Vax.
Pakistan. The bulk vaccine from CanSino is formulated, sterilized, and packed at a filling plant in the National Institute of Health Pakistan, and a team from China helped the institute set up the production operation. Local media reported that locally filling vaccine vials will reduce the price of the vaccine by up to 30 percent, and that the CanSino vaccine will be manufactured in Pakistan through technology transfer at a later stage.
Bangladesh. The country’s high officials also vowed to secure vaccines from all possible sources to meet its needs, and in light of the decision, the Cabinet Committee on Economic Affairs approved a proposal on locally producing China’s Sinopharm vaccine.
Serbia. Construction of the factory in Serbia will be co-financed by Chinese and the UAE, while Serbia will contribute the land.
Turkey. A report by the Turkish media says that Sinovac, the Chinese producer of its COVID-19 vaccine CoronaVac, has provided a license to Turkey to manufacturing the jabs. Turkey is among five countries given a production license for the vaccine, along with Indonesia, Brazil, Malaysia, and Egypt.
Argentina. China’s Sinopharm and an Argentina pharmaceutical company have reached an agreement to produce vaccines in Argentina with discussions on technology transfer to follow, aiming to begin production as soon as possible.
Brazil. The state-run Butantan Institute led mass clinical testing of the Sinovac vaccine in Brazil and is now filling and finishing Sinovac vaccines in Brazil, with plans for 100% domestic production in early 2022. Butantan Institute is also building a facility to produce 100 million doses a year of China’s Sinovac COVID-19 vaccine, which will be ready by September 2021.
Mexico. CanSino exports active ingredient for its vaccines to Mexico, which is then packaged in Mexico by Drugmex, CanSino said in a statement. CanSino stated that it expects to produce 6.9 million doses between March and June 2021, and then make 1.2 million shots available per week to fulfill its agreement for 35 million doses this year.
Notes
Vaccine procurements:
Some of the vaccine procured by Brazil, Indonesia, Malaysia, and Mexico will be produced locally from active ingredients delivered by China.
The Chinese military has also donated vaccines to the military in Cambodia, Congo Republic, Mongolia, Pakistan, the Philippines, Sudan, North Macedonia, Zimbabwe, Guinea, and Tunisia; some quantities are included in the reported data above, while others are undisclosed.
Turkmenistan received vaccines from China, but quantities were undisclosed.
Argentina’s total purchased quantity of Sinopharm vaccines remains undisclosed.
Thailand’s total purchased quantity might be higher than recorded, as certain numbers were undisclosed.
China has offered vaccines to Nepal under a “grant assistance”, which is not a complete donation.
Iraq and Kazakhstan have received vaccines that are assumed to be purchased from China.
Additional procurements:
The UAE has donated vaccines to Seychelles, Jordan, Belize, Paraguay, and Indonesia; the quantities are recorded above.
Turkey has provided vaccines to Libya, North Macedonia, and Azerbaijan; the quantities are recorded above.
Chile has donated vaccines each to Ecuador and Paraguay; the quantities are recorded above.
One million Sinopharm doses will be used in Indonesia’s private vaccination program known as Gotong Royong.
Malaysia’s state of Sarawak has procured Sinovac doses, but it is unclear as to whether they were independently procured.
